6 December 2013
In many ways, traditional chemical synthesis is similar to cooking. To alter the final product, you can change the ingredients or their ratio, change the method of mixing ingredients, or change the temperature or pressure of the environment of the ingredients. Like an accomplished chef, chemists have become very skilled at the manipulation of these parameters to produce many of the products that make our lives better.
But there are some things that resist these methods. As a result, researchers are continually looking for new techniques to apply. In particular, laser-based chemistry has been a goal for researchers since the invention of the laser in the 1960s. Applying a laser pulse of the correct color and duration to a molecule could, in principle, inject just the right amount of energy to modify a specific chemical bond and change the molecule into a more desirable configuration. In this sense, the laser can be thought of as a new type of reagent that drives a chemical reaction.
In practice, even a single molecule is a complicated system and finding the correct laser pulse characteristics to influence molecules is difficult. In addition, sophisticated laser pulse shaping devices can produce a nearly infinite number of pulse shapes, making a systematic search for the correct laser-molecule solution daunting.
A proven method for approaching this problem is to use experimental feedback to guide an adaptive search of the possible laser pulses. As in natural selection, laser pulses that provide a better outcome are given an increased chance to survive and have their characteristics contribute to the tailored pulse that ultimately produces the desired outcome. Such a method, however, is only as good as the feedback that drives it.
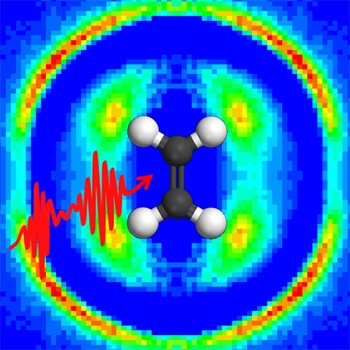
In an article published this week in the journal Nature Communications, researchers from Augustana College (SD) and Kansas State University (KSU) in the United States and from the Max Planck Institute for Quantum Optics (MPQ) and the Ludwig Maximilian University (LMU) in Munich, Germany, have reported an improved feedback technique. By imaging the dissociating molecule in three dimensions, a laser pulse can be optimized to drive the molecule to a very specific final state. This image-based technique can complement feedback methods that depend on optical spectroscopy. Furthermore, the researchers were able to use the dissociation images to guide theoretical work that revealed how the laser pulse was able to control the molecule, in this case driving acetylene ions from the normal HCCH configuration to the unusual HHCC configuration.
Building on the initial work done at MPQ, Augustana students developed a method for converting the image into feedback quickly enough to be useful in the experiment. They then developed a system of computer control linking the entire experiment as well as refining image-analysis techniques to evaluate the experimental data. Once this was accomplished, the experiment was conducted at the J.R. Macdonald Laboratory. Initial results stimulated the theoretical work performed at LMU to clarify the control mechanism.
“The experiment shows that improved feedback, provided by multi-dimensional imaging, enhances both our abilities to control chemical reactions and the physical insight that can be gained”, said Matthias Kling, research group leader at MPQ and assistant professor at KSU at the time the studies were conducted. “The new methodology provides new possibilities for the control of more complex systems including larger molecules, clusters, and nanoparticles. Multi-dimensional data provide stricter limitations for theory and will help to improve our models”, explains Regina de Vivie-Riedle, professor at LMU and leader of the group that performed the theory.
Augustana College personnel and equipment were funded by National Science Foundation Grant No. 0969687 and National Science Foundation/EPSCoR Grant No. 0903804. KSU operations and personnel were supported by the Chemical Sciences, Geosciences and Biosciences Division, Office of Basic Energy Sciences, Office of Science, U.S. Department of Energy. Additional funding was provided by support by the German Research Foundation via the Cluster of Excellence: “Munich Center for Advanced Photonics (MAP)” and via the DFG grants Kl-1439/2 and Kl-1439/3.
Original publication:
E. Wells, C.E. Rallis, M. Zohrabi, R. Siemering, Bethany Jochim, P.R. Andrews, U. Ablikim, B. Gaire, S. De, K.D. Carnes, B. Bergues, R. de Vivie-Riedle, M.F. Kling, and I. Ben-Itzhak
Adaptive Strong-field Control of Chemical Dynamics Guided by Three-dimensional Momentum Imaging
Nature Communications 4:2895 DOI: 10.1038/ncomms3895 (2013).