Irvine, Calif., Sept. 16, 2014 – UC Irvine chemists have scored a scientific first: capturing moving images of a single molecule as it vibrates, or “breathes,” and shifts from one quantum state to another.
The groundbreaking achievement, led by Ara Apkarian, professor of chemistry, and Eric Potma, associate professor of chemistry, opens a window into the strange realm of quantum mechanics – where nanoscopic bits of matter seemingly defy the logic of classical physics.
This could lead to a wide variety of important applications, including lightning-fast quantum computers and uncrackable encryption of private messages. It also moves researchers a step closer to viewing the molecular world in action – being able to see the making and breaking of bonds, which controls biological processes such as enzymatic reactions and cellular dynamics.
The August issue of Nature Photonics features this breakthrough as its cover story.
“Our work is the first to capture the motion of one molecule in real time,” Apkarian said. While still images of single molecules have been possible since the 1980s, recording a molecule’s extremely rapid movements had proven elusive.
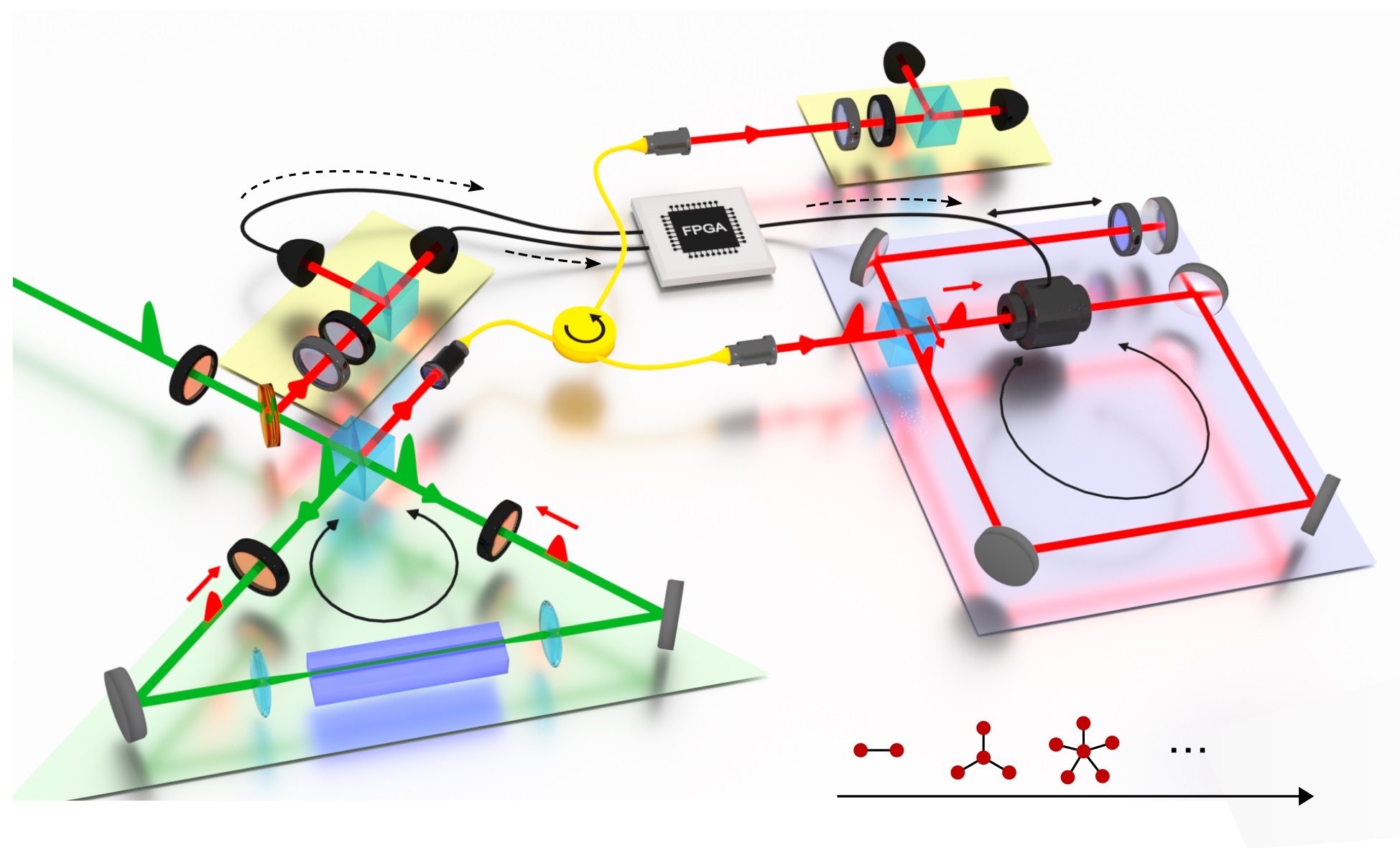
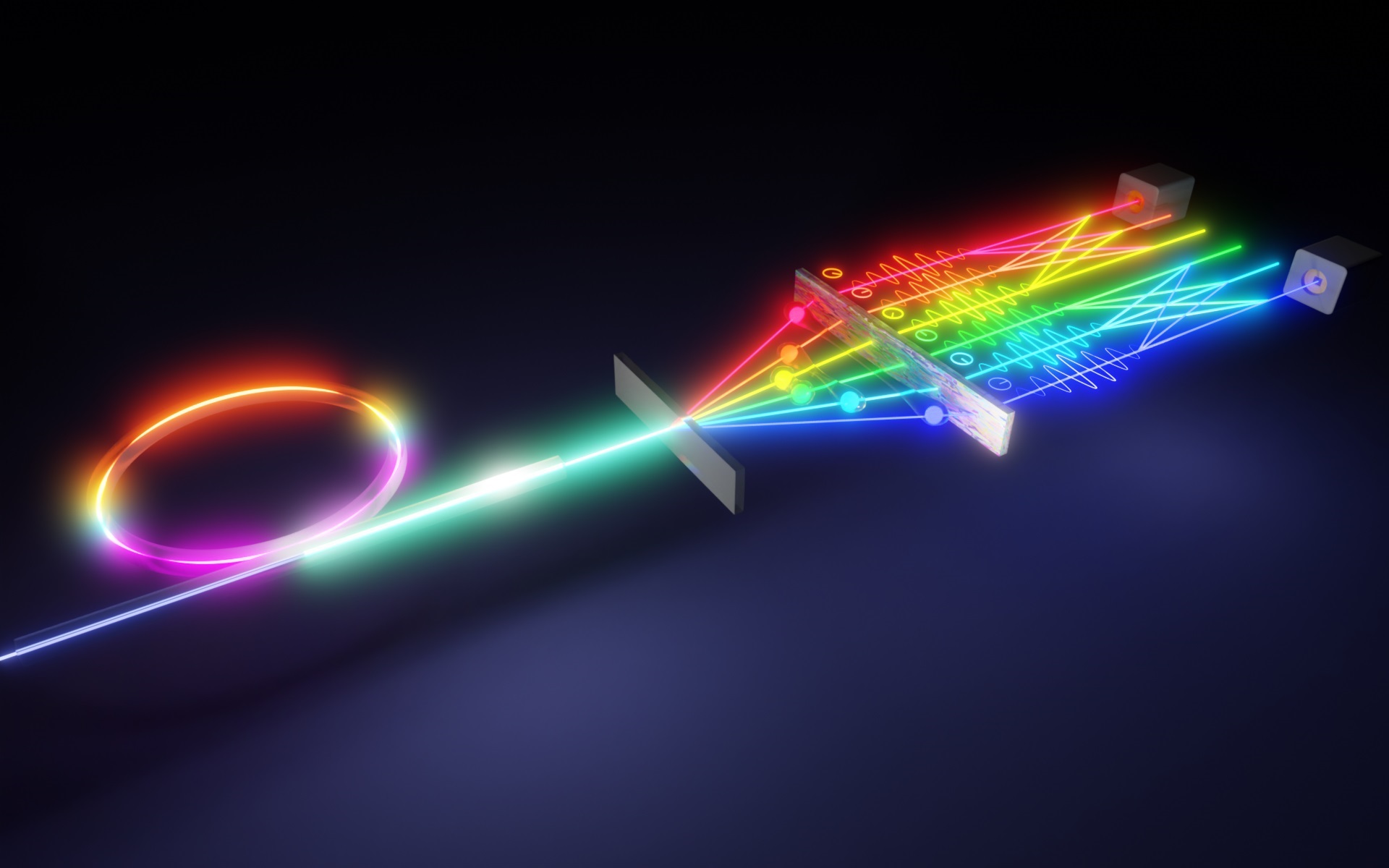
In addition to using precisely tuned, ultrafast lasers and microscopes, the researchers had to equip the molecule with a tiny antenna consisting of two gold nanospheres in order to track its activity and record measurements over the course of an hour.
When the many repeated measurements were averaged, an astonishing finding emerged: The molecule was oscillating from one quantum state to another.
The scientists have produced a movie in which a small, glowing dot appears to emit pulses of bright light. “That’s the light broadcast from the antenna every time the molecule completes a cycle of its vibrational motion,” Apkarian said. “The bond moves at a rate of 1013 cycles per second – a million, million times 10 cycles in one second.” Making the movie was like freeze-frame photography with a very fast flash and repeating the measurement over and over again.
Seeing a molecule as it moves is “essential to a deeper understanding of how it forms and breaks chemical bonds,” Potma said. “The aim of the present experiment was to demonstrate that we can capture a molecule in motion on its own timescale.”
The next and even more ambitious goal is to acquire moving images of molecules in their natural environment without tethering them to an antenna. “Ultimately, we’d like to be able to [examine] a molecule … as it’s undergoing chemistry,” Apkarian said.
He and Potma are members of the Center for Chemistry at the Space-Time Limit, a research entity funded by the National Science Foundation that involves about 60 scientists on five campuses.
(Any opinions, findings, conclusions or recommendations expressed in this material are those of the study authors and do not necessarily reflect the views of the NSF).