September 11, 2014
By Bjorn Carey
By observing how hydrogen is absorbed into individual palladium nanocubes, Stanford materials scientists have detailed a key step in storing energy and information in nanomaterials. The work could inform research that leads to longer-lasting batteries or higher-capacity memory devices.
The ideal energy or information storage system is one that can charge and discharge quickly, has a high capacity and can last forever. Nanomaterials are promising to achieve these criteria, but scientists are just beginning to understand their challenging mechanisms.
Now, a team of Stanford materials scientists and engineers has provided new insight into the storage mechanism of nanomaterials that could facilitate development of improved batteries and memory devices.
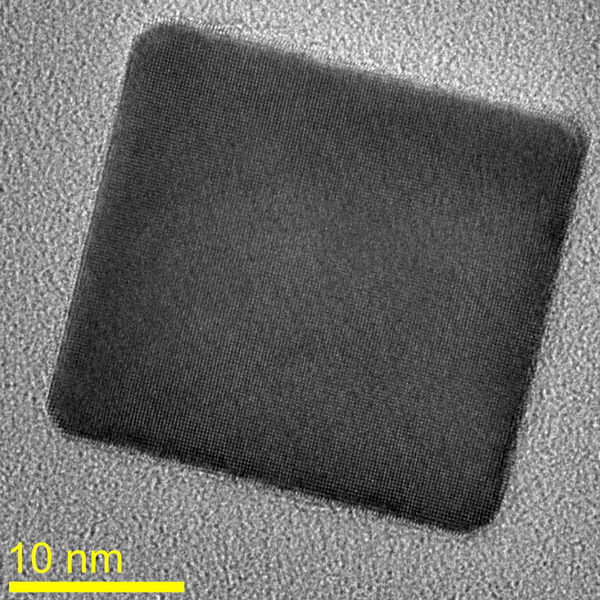
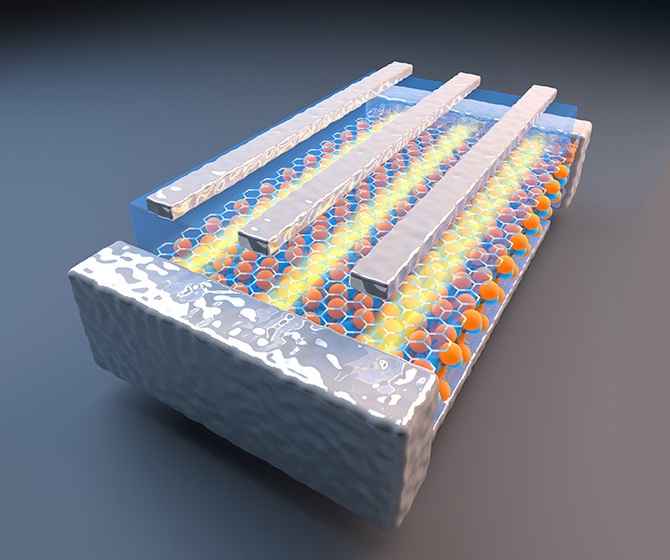
The team, led by Jennifer Dionne, assistant professor of materials science and engineering at Stanford, and consisting of Andrea Baldi, Tarun Narayan and Ai Leen Koh, studied how metallic nanoparticles composed of palladium absorbed and released hydrogen atoms.
Previously, scientists have studied hydrogen absorption in ensembles of metallic nanoparticles, but this approach makes it difficult to infer information about how the individual nanoparticles behave. The new study reveals that behavior by measuring the hydrogen content in individual palladium nanoparticles exposed to increasing pressures of hydrogen gas.
The group's experimental findings are consistent with a mechanism recently proposed for energy storage in lithium ion batteries, underscoring the interest for the broader scientific community. The work is detailed online in the journal Nature Materials.
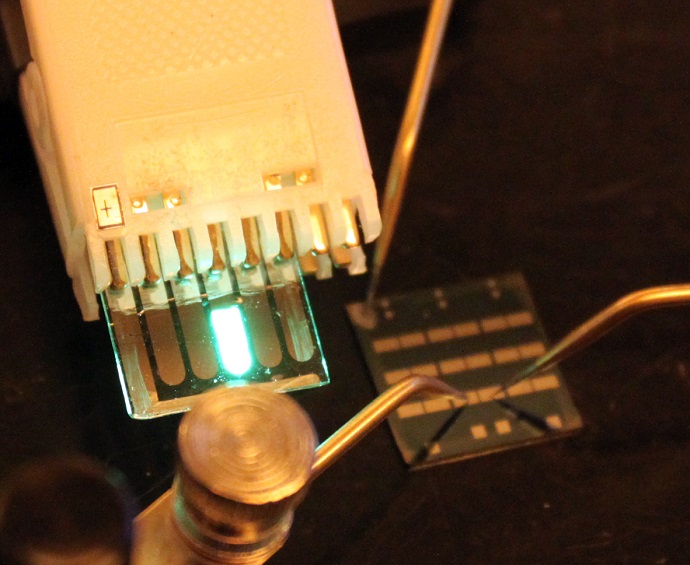
The finding was made possible by the use of a specialized transmission electron microscope (TEM) that allowed the team to detect, with near atomic-scale resolution, the process by which hydrogen entered the nanomaterial.
"Electron microscopy must ordinarily be conducted in high vacuum," said co-author Ai Leen Koh, a research scientist with the Stanford Nano Shared Facilities. "But the unique capabilities of Stanford's environmental TEM obviates this requirement, enabling the study of individual nanoparticles both in vacuum and while immersed in a reactive gas."
Stretching metal
The researchers synthesized palladium nanocubes and then dispersed them onto a very thin membrane. After placing the membrane in the TEM, the engineers flowed hydrogen gas past the palladium nanoparticles and gradually increased its pressure.
At sufficiently high pressures of hydrogen, the gas molecules dissociate on the surface of the nanocubes and individual hydrogen atoms enter into the spaces between the palladium crystals. Interestingly, the absorption and desorption processes appear to be quite sudden.
"You can think of it like popcorn," said co-lead author Tarun Narayan, a graduate student in Dionne's group. "It's a very binary process, and a pretty sharp transition. Either the hydrogen is in the palladium or it's not, and it enters and leaves at predictable pressures. And that's quite important for a good energy storage system."
As the hydrogen enters the palladium nanostructure, the material's volume increases by about 10 percent. This expansion significantly alters the way in which the particle interacts with the electron beam; this disruption indicates the amount of hydrogen absorbed. Because the nanocubes are single-crystalline and effectively "unbound" from the membrane, the researchers were able to study and measure the storage mechanism in unprecedented detail.
"You have to stretch the palladium to put the hydrogen inside, but you have to pay energy to make it stretch," said Andrea Baldi, a postdoctoral researcher in Dionne's group. "Knowing that cost is very important for any battery designs, and because our nanostructures are not glued to a substrate, we're able to quantify that stretch more accurately than ever before."
Next up: Palladium spheres
Despite the stress of repeated expansion and contraction, the nanocrystals of palladium were not damaged by hydrogen absorption and desorption, as usually happens in larger specimens.
"At the nanoscale, materials behave quite differently than they do in bulk," said Dionne, the senior author. "Their increased surface area to volume ratio can significantly impact their mechanical flexibility and, consequently, their ability to charge and discharge ions or atoms."
In particular, this research indicates that nanoparticles can load more easily and at much lower pressures than bulk materials. Further, because they have a higher resistance to elastic stress, the formation of defects in these materials is suppressed.
"Our results suggest that particles in this size regime don't develop defects even if charged and discharged with hydrogen multiple times," Narayan said. "Other researchers are starting to see this in lithium ion battery research as well, and we think a lot of what we've learned can be applied to that research."
Because of its fast storage speeds, stability and ease-of-loading, the hydrogenation of palladium is an excellent model system to study general energy and information storage mechanisms. Palladium, however, is not a likely material for widespread energy storage – it is too heavy and expensive. Yet, the researchers believe the results could be replicated with other systems involving storing hydrogen in metals.
The next steps involve applying the newly developed single-particle method to a wide range of nanostructures – spheres and rods, for example – to study how storage can be affected by the shape, size and crystallinity of a nanoparticle. Furthermore, they plan to use the electron microscope to determine exactly where atoms or ions are preferentially absorbed within a singe nanoparticle.
Dionne is an affiliate of the Stanford Institute for Materials and Energy Sciences (SIMES) and SLAC National Accelerator Laboratory. The research was supported by funding from the National Science Foundation, the Air Force Office of Scientific Research, the U.S. Department of Energy, a Young Energy Scientist Fellowship and a Hellman Fellowship.