June 26, 2017
Unless you have unlimited funds, finding solar power that is affordable and highly efficient is largely out of reach. A Michigan State University chemist is hoping to change that.
Thomas Hamann, the James L. Dye Chair in Materials Chemistry, intends to use a $465,000 grant from the U.S. Department of Energy to create a new paradigm for capturing solar energy.
“We’re capable of making photovoltaics that are close to 50 percent efficient, but only NASA can afford those,” Hamann said. “Little changes to existing technology won’t cut it; we need to make paradigm changes and discover new approaches.”
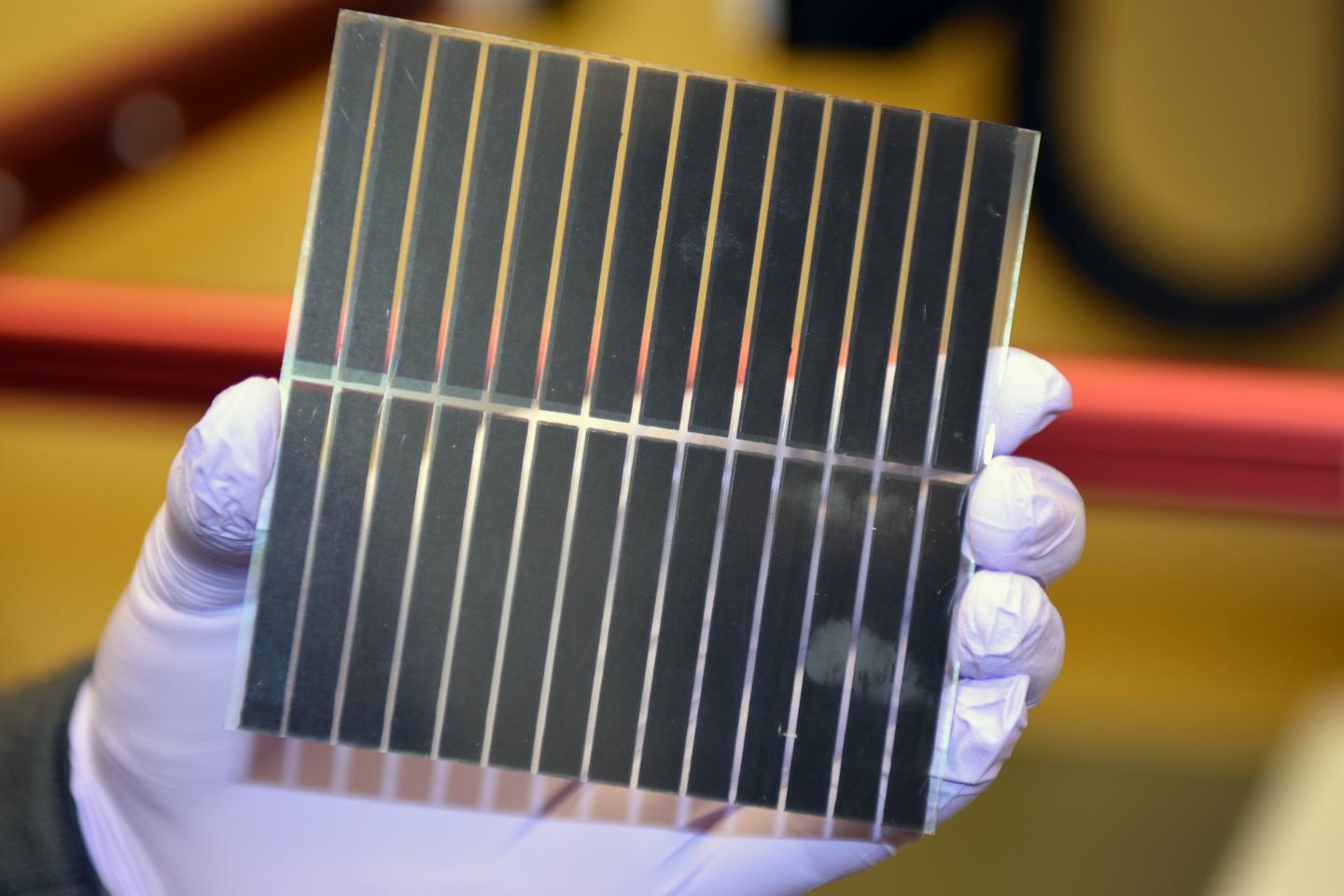
Hamann is working on a platform called a dye-sensitized solar cell that is potentially quite cost effective. His big challenge is to make them more efficient. These solar cells comprise three components: a semiconductor; dye, which acts as the light absorber; and redox molecules that shuttle electrons. Hamann is exploring new materials for semiconductors as well as new redox molecules.
“We’re making new redox molecules and combining them with various semiconductors to understand and control recombination – the back reaction that generally limits the efficiency of solar cells,” Hamann said. “There’s probably been more than 1,000 dyes tested, but little research has focused on developing new materials or redox molecules.”
The best dye-sensitized solar cells achieve more than 10 percent solar-to-electricity power conversion efficiency. They fall short of their 20-percent potential level largely because of energy lost in each electron-transfer step, Hamann said.
One way Hamann and his team are tackling this issue is by developing new cobalt-based redox shuttles. By changing the ligands that bind to the cobalt center, he has shown they can control the rate of electron transfer by a factor of a billion. Combined with the ability to modify the redox potential, this paradigm offers to possibility to quickly transfer electrons where they are needed, but not suffer from recombination.

“All redox shuttles investigated to date have been generally constrained to a small potential window in order to minimize the driving force of dye regeneration with optimized, existing dyes,” Hamann said. “This energy constraint limits the ability to fully understand and optimize the kinetics.”
By elevating redox shuttles into the research spotlight, Hamann hopes to open up new paths to improved performance through kinetic manipulation. He’s also looking forward to the new science this will spur.
“Our ultimate goals are to develop a next-generation solar cell as well as eventually find a way to convert it to a carbon-free liquid fuel,” Hamann said. “Solving the conundrum of finding renewable energy has always been a goal of my research. I enjoy attempting to solve important problems, and I believe we can find sustainable approaches, build new transmission lines and develop new storage batteries to solve these issues.”