08/12/2015
Yale researchers have significantly increased the efficiency of a polymer solar cell by using a technique that mimics how plants use solar energy and forcing two otherwise incompatible molecules to work together to cover the full color spectrum.
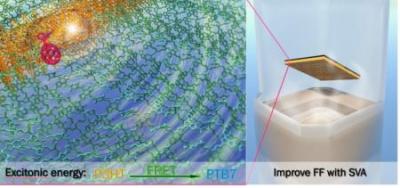
The researchers, in Dr. Andre Taylor’s Transformative Materials & Devices Lab, developed a solar cell that performed 22.5 percent better than conventional organic solar cells. Their results were published online this month in the Journal of Materials Chemistry A demonstrating a power conversion efficiency of 8.7 percent.
Most commercial solar cells today are made from silicon. But polymer cells cost less and weigh less, making them an appealing alternative. The problem is that they’re not very efficient – they fail to convert nearly half their absorbed light energy to electrical power. That’s partly because the polymers used in these cells don’t line up well enough to allow energy to exit the cell easily.
However, because polymers have a mechanical flexibility that silicon cells don’t, researchers are hopeful that they will find ways around these shortcomings.
“We are starting to approach the limits for improvements that can done with conventional silicon solar cells,” Taylor said. “But with organic polymers you can tweak and do things to them with significant results.”
In a 2013 study in Nature, Taylor’s lab was the first to show that this can occur between small molecules and a polymer known as P3HT. It’s now demonstrating some of those same benefits in polymer blends.
Conventional organic solar cells, known as binary solar cells, have one polymer serving as an electron donor and a fullerene derivative as the electron acceptor. Ternary cells – the kind used in this study - can have either two donors and one acceptor or one donor and two acceptors. In most cases, though, more efficient ternary cells usually have two donors and one acceptor since donors are predominantly responsible for light absorption.
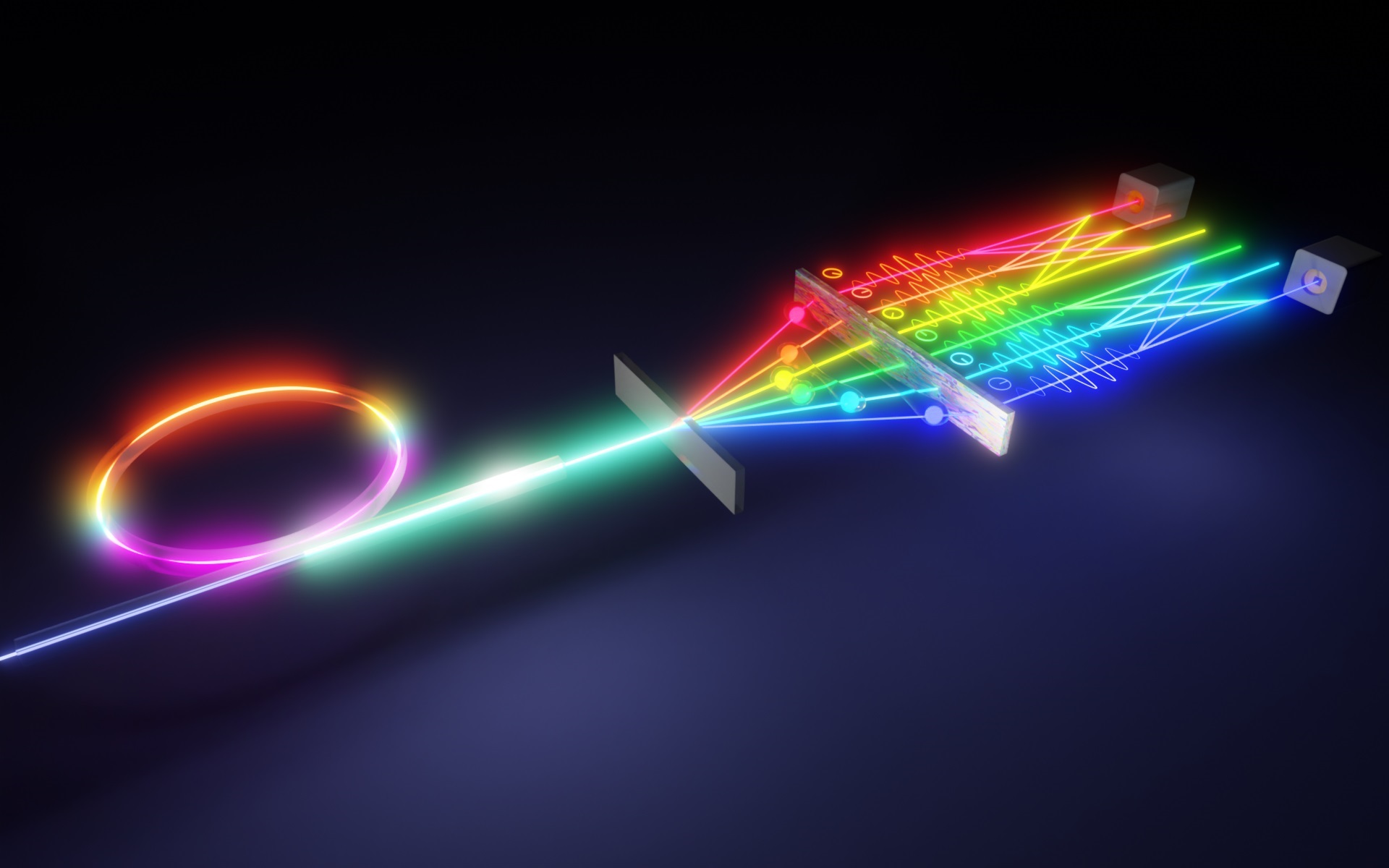
The most recent study uses two polymers, P3HT and PTB7, which are both light-sensitive molecules known as chromophores. In one sense, the two are complementary: P3HT absorbs the blue-green side of the light spectrum, while PTB7 absorbs primarily at the yellow-red spectrum. Together, the two cover a large portion of the visible-light spectrum. Rather than working independently, the proximity of the two polymers also facilitates what’s known as Förster resonance energy transfer (FRET) to occur. That’s when energy is transferred between two chromophores over long distances.
The problem is how these two polymers align.
“We are blending two different types of polymers, so they align in different ways,” said TengHooi Goh, lead author of the paper. “P3HT aligns in a way that it stands like a wall and PTB7 is positioned more like a stack of pancakes.”
“They work well optically, but the contradicting alignment is bad for electron transport,” added Taylor, senior author of the paper.
To get around this problem, the researchers used a technique known as solvent vapor annealing (SVA), in which they chemically modify the properties of the polymers to better align. The more commonly used method is thermal annealing, but heat has been found to diminish the performance of the polymers. Goh said that SVA can potentially solve incompatible alignment problems in complex polymer systems and drive the efficiency of organic photovoltaics to a new heights.
The other authors of the paper, “Panchromatic Polymer-polymer Ternary Solar Cells Enhanced by Förster Resonance Energy Transfer and Solvent Vapor Annealing,” are Jing-Shun Huang, Benjamin Bartolome,Matthew Y. Sfeir, Michelle Vaisman, and Minjoo Lee.